It is the time to bring more excitement to science. Learn the new concepts and grab these conveniently. The process of learning concepts with the assistance of comparison is the ideal one. Let us view how diffusion differs from osmosis and vice versa:
Osmosis VS Diffusion
Solute Concentration
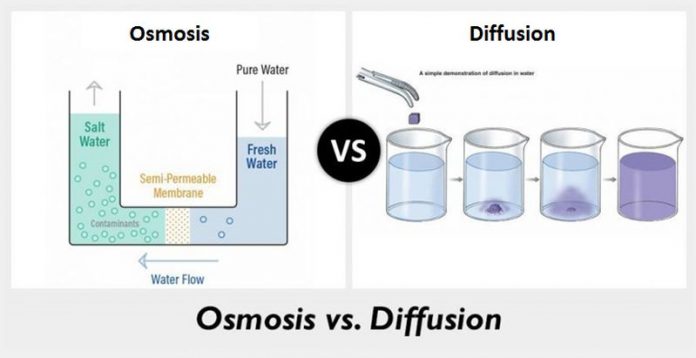
The central aspect that differentiates diffusion from osmosis is concentration. In osmosis, there are solute molecules that reflect their movement from the zone or area of low concentration. They start moving from low concentration to the area or zone of high concentration. The process of osmosis is the one that is possible to avoid through applying pressure to it.
However, diffusion is when the solute molecules reflect their movement from the zone of high concentration to the zone or area of low concentration. No matter how much pressure apply, the process of diffusion cannot avoid.
Medium
Osmosis is a process that cannot carry out in multiple mediums. It requires only the liquid medium for its execution. However, in the case of diffusion, there is no limitation of the medium. The diffusion process can occur in all the mediums, such as gas, liquid, and gas. Hence, diffusion is found to more versatile than osmosis.
Membrane
The process of osmosis cannot occur in any membrane. It demands only the semipermeable membrane for its process. In comparison, diffusion has the excellent capability to execute its process without the requirement of any semipermeable membrane.
Direction
It is a confusing point for a majority of the students. However, it is a significant question from an exam point of view. Hence, one must remember the direction of diffusion and osmosis. Osmosis is a process that occurs merely in one direction, and hence it is also known as unidirectional. While diffusion varies from this, it can occur quickly in all directions and does not limit its direction. The movement of the particles for the diffusion can see in any direction.
Speed: Difference between osmosis and diffusion
The speed of both of these processes varies from each other. Osmosis and diffusion do not process at the same speed. Indeed, one of these occurs at a much faster rate while the other one takes much time. Osmosis is a time-consuming process and takes much time to accomplish. It is papular as a slow process, while diffusion is found to be opposite to this one. Diffusion occurs faster and is hence accomplished quietly soon, and the particle starts moving throughout the medium without following any particular direction.
Also read: Difference between formal and informal letter
Particle Movement
There is a movement of a particle in both the diffusion and osmosis process. However, the area covered by the particles varies for both processes. In the case of osmosis, solute particles move amazingly and cover a considerable distance. The movement of the solute particles follows a specific direction and spreads quite extensively. However, for the discussion process, the scenario is different. The solute particles in diffusion move the short distance only and can found near to each other. They do not cover huge distances but can found close to each other.





order atorvastatin 40mg pills lipitor 20mg drug cost atorvastatin 80mg
ciprofloxacin order online – buy cipro 500mg augmentin 625mg usa
ciprofloxacin for sale online – baycip pills cost augmentin 625mg
ciprofloxacin 500 mg for sale – buy tinidazole pills for sale buy erythromycin 250mg pill
metronidazole 200mg ca – brand oxytetracycline 250 mg buy zithromax no prescription
ivermectin 3 mg otc – tetracycline 250mg cost order tetracycline for sale
order valtrex 1000mg – nemasole for sale order acyclovir 800mg generic
cheap metronidazole 200mg – flagyl for sale online order azithromycin generic
lasix 40mg usa – buy cheap prazosin buy capoten 25 mg pills
cost metformin – order cefadroxil 250mg sale order lincomycin for sale
buy zidovudine 300 mg pills – order zyloprim 100mg
order clozaril 50mg generic – ramipril 10mg oral famotidine over the counter
buy anafranil generic – doxepin 75mg brand purchase sinequan
seroquel price – buy effexor 150mg sale eskalith without prescription
buy generic hydroxyzine 25mg – pamelor 25 mg over the counter buy endep 25mg online
buy augmentin generic – buy ciprofloxacin tablets buy cipro pill
purchase amoxil generic – buy generic axetil for sale where to buy cipro without a prescription
purchase azithromycin sale – buy ciplox paypal buy ciplox 500mg generic
order cleocin 300mg pill – order cleocin 300mg generic purchase chloramphenicol for sale
ivermectin 3 mg for sale – buy eryc 500mg buy cefaclor online cheap
albuterol 4mg ca – seroflo without prescription theo-24 Cr order online
methylprednisolone 4 mg otc – claritin over the counter buy astelin 10 ml without prescription
buy desloratadine pill – order generic albuterol albuterol for sale online
metformin cheap – order glycomet pills precose price
glyburide 2.5mg without prescription – order glyburide 5mg online cheap dapagliflozin pill